Diazyme's Direct HbA1c (Enzymatic, On-Board Lysis) is a unique single channel assay ideal for labs requiring a high throughput HbA1c method with no off-line pretreatment steps. High throughput is obtained using a patented single channel method which eliminates the need for a dedicated channel for total hemoglobin, thereby improving assay turnaround time and providing the added convenience of instrument specific packaging options.
| Product | Catalog Number | Format | Method |
|---|---|---|---|
| Kit | DZ168C | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Enzymatic |
| Calibrator | DZ168C-CAL | Cal: 2 Level | |
| Control | DZ168C-CON | Con: 2 Level |
Product Features
Diazyme's Direct HbA1c (Enzymatic, On-Board Lysis) is a unique single channel assay ideal for labs requiring a high throughput HbA1c method with no off-line pretreatment steps. High throughput is obtained using a patented single channel method which eliminates the need for a dedicated channel for total hemoglobin, thereby improving assay turnaround time and providing the added convenience of instrument specific packaging options. The assay has a wide AMR range of 4.0% - 12.0% HbA1c. The assay offers enhanced precision and is resistant to interference from variant hemoglobin and post transcript modifications which can impact the accuracy of other HbA1c assays.
Assay Principle
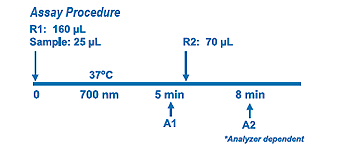
Diazyme Direct HbA1c Assay is an enzymatic assay in which lysed whole blood samples are subjected to extensive protease digestion with Bacillus sp protease. This process releases amino acids including glycated valines from the hemoglobin beta chains. Glycated valines then serve as substrates for specific recombinant fructosyl valine oxidase (FVO) enzyme. The recombinant FVO specifically cleaves N-terminal valines and produces hydrogen peroxide. This, in turn, is measured using a horseradish peroxidase (POD) catalyzed reaction and a suitable chromogen. No separate measurement for total Hemoglobin (Hb) is needed in this Direct HbA1c Assay. The HbA1c concentration is expressed directly as %HbA1c by use of a suitable calibration curve in which the calibrators have values for each level in %HbA1c.
Intended use
The Diazyme Direct HbA1c Assay (Enzymatic, On-Board Lysis) test kit is intended for use in the quantitative determination of stable HbA1c in venous whole blood samples with on-board blood lysis application in a clinical laboratory. This test is not to be used to diagnose or screen for diabetes. The measurement of HbA1c concentration is for use in monitoring long-term glucose control of persons with diabetes. For in-vitro diagnostic use only.
Regulatory Status

 Health Canada Registered
Health Canada Registered

