Diazyme's Apo B Assay has a linear range of 3.6 – 240 mg/dL with good correlation with existing FDA approved product with r2 values >0.99. The Diazyme Apo B Assay is not interfered by the following substances at the indicated concentrations: triglycerides at 1000 mg/dL, ascorbic acid at 10 μM, bilirubin at 40 mg/dL, bilirubin conjugate at 40 mg/dL, and hemoglobin at 1000 mg/dL.
| Product | Catalog Number | Format | Method |
|---|---|---|---|
| Kit | DZ142A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ141A-CAL | Cal: 1 Level (Lyophilized) | |
| Control | DZ248A-CON | Con: 3 Level (Lyophilized) |
Product Features
Diazyme's Apo B Assay has a linear range of 3.6 – 240 mg/dL with good correlation with existing FDA approved product with r2 values >0.99. The Diazyme Apo B Assay is not interfered by the following substances at the indicated concentrations: triglycerides at 1000 mg/dL, ascorbic acid at 10 μM, bilirubin at 40 mg/dL, bilirubin conjugate at 40 mg/dL, and hemoglobin at 1000 mg/dL.
Downloads
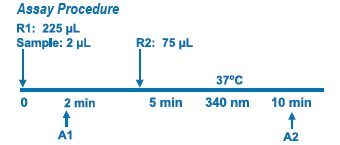
Assay Principle
This method is based on the reaction of a sample containing human Apo B and a specific antiserum to form an insoluble complex which can be measured turbidimetrically at 340 nm. By constructing a standard curve from the absorbance of standards the concentration of apo B in the samples can be determined.
Intended use
The Diazyme Apolipoprotein B Assay is intended for the quantitative in vitro determination of Apolipoprotein B (Apo B) in serum. It can be used as an aid for assessing the risk of coronary artery disease. For in vitro diagnostic use.
Regulatory Status

 Health Canada Registered
Health Canada Registered

