Myeloperoxidase (MPO)

 |
| Product Features
The linearity of the assay is from 12 – 1000 ng/mL (83 – 6940 pM) in heparin plasma with intra-assay % CV ≤5%. The following substances normally present in the plasma were tested at levels equal to the following concentrations. Ascorbic acid (10mM), bilirubin (free) (40 mg/dL), conjugated bilirubin (40 mg.dL), triglycerides (270 mg/dL), Hemoglobin (200 mg/dL), Rheumatoid Factor (75 IU/mL) produced less than 10% deviation when tested. |
|||
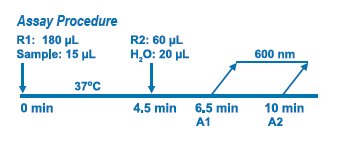 Assay Principle Assay Principle
Diazyme's MPO Immunoassay is based on a latex enhanced immunoturbidimetric assay. MPO proteins in the sample bind to the specific anti-MPO antibody, which is coated on latex particles, and causes agglutination. The degree of the turbidity caused by agglutination can be measured optically and is proportional to the amount of MPO in the sample. The instrument calculates the MPO concentration of a patient specimen by interpolation of the obtained signal of a 6-point calibration curve. |
|||
| Intended use
Diazyme MPO Immunoassay is for the quantitative determination of myeloperoxidase in plasma by latex enhanced immunoturbidimetric method. For Research Use Only in USA. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ178C | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ178C-Cal | Cal: 5 Level | |
| Control | DZ178C-Con | Con: 2 Level | |
| Regulatory Status
|
|||


