Homocysteine 2 Reagent Enzymatic Assay
 |
Homocysteine (HCY) Informational Articles
| Product Features
Diazyme's Homocysteine (HCY) 2 Reagent Enzymatic Assay is a convenient dual vial liquid stable assay ideal for analyzers which require two-part reagent systems. The assay provides a fast, accurate cost-effective alternative to cumbersome and expensive immunochemical methods. Accurate and reliable, the assay features excellent correlation with HPLC and immunochemical methods with a linear range of 3-50 μmol/L and intra and inter %CV values of < 7.5%. The assay has no significant interference from lipemic and hemolytic substances and eliminates the "carry-over" interference with iron or lipase reagents that can occur with other methods. More significantly, the assay is not interfered by the endogenous cystathionine and therefore can be used with confidence in patients with renal disease. |
||
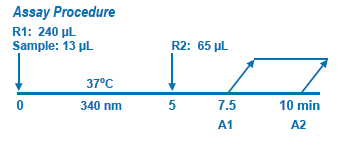 Assay Principle Assay Principle
In Diazyme's Hcy Assay, oxidized Hcy is first reduced to free Hcy which then reacts with a co-substrate, S-adenosylmethionine (SAM) catalyzed by a Hcy S-methyltransferase to form methionine (the Hcy conversion product of Hcy) and S-adenosylhomocysteine (SAH, the co-substrate conversion product). SAH is assessed by coupled enzyme reactions including SAH hydrolase, adenosine (Ado) deaminase and glutamate dehrogenase, where-in SAH is hydrolyzed into adenosine (Ado) and Hcy by SAH hydrolase. The formed Hcy that is originated from the co-substrate SAM is cycled into the Hcy conversion reaction by Hcy S-methyltransferase. The formed Ado is immediately hydrolyzed into inosine and ammonia. In the last step, the enzyme glutamate dehydrogenase (GLDH) catalyzes the reaction of ammonia with 2-oxoglutarate and NADH to form NAD+. |
||
| Intended use
The Diazyme Homocysteine 2 Reagent Enzymatic Assay kit is intended for the in vitro quantitative determination of total L-homocysteine in serum or plasma. The assay can assist in diagnosis and treatment of patients suspected of having hyperhomocysteinemia and homocystinuria. Patients who are taking methotrexate, carbamazepine, phenytoin, nitrous oxide, anticonvulsants, or 6-azuridine triacetate may have higher levels of Hcy due to metabolic interference with Hcy metabolism. |
||
| Ordering Information | ||
| Product | Catalog Number | Format |
| Kit | DZ568B | R1/R2 (Dual Vial Liquid Stable, Enzyme Cycling) |
| Calibrator | DZ568A-CA5 | Cal: 5 Level |
| Control | DZ568A-CON | Con: 4 Level |
| Regulatory Status
|
||



