D-Dimer

 |
|
| Product Features
Diazyme's D-Dimer Assay is a highly sensitive liquid stable latex enhanced immunoturbidimetric test kit, designed to work on most open clinical chemistry analyzers to provide rapid results with reduced reagent cost. The D-Dimer Assay has a linearity of 0.15 - 8.0 μg/mL FEU with a correlation coefficient value of 0.939, slope of 0.979 and y intercept of -0.106. The following substances do not interfere with this assay at the levels tested less than 10% bias: Hemoglobin up to 500 mg/dL, Bilirubin 40 mg/dL, Bilirubin conjugated 40mg/dL, Triglycerides 1000 mg/dL, Ascorbic Acid 176 mg/dL, Rheumatoid Factor 100 IU/mL, Heparin 1.5 IU/mL and HAMA 490 ng/mL. |
|||
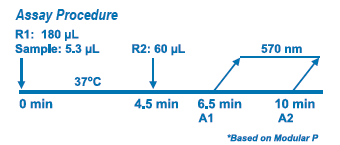 Assay Principle Assay Principle
Diazyme's D-Dimer Assay is based on a latex enhanced immunoturbidimetric assay. D-Dimer proteins in the sample bind to the specific anti-D-Dimer antibody, which is coated on latex particles, and causes agglutination. The degree of the turbidity caused by agglutination can be measured optically and is proportional to the amount of D-Dimer in the sample. The Roche™ Modular P calculates the D-Dimer concentration of a patient specimen by interpolation of the obtained signal of a 6-point calibration curve. |
|||
| Intended use
Diazyme's D-Dimer Assay is for the quantitative determination of fibrinogen/fibrin degradation products (D-Dimer) in human plasma. Measurement of D-Dimer is used as an aid in detecting the presence of intravascular coagulation and fibrinolysis. For in vitro diagnostic use only. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ179A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ179A-Cal | Cal: 5 Level (Lyophilized) | |
| Control | DZ179A-Con | Con: 2 Level (Lyophilized) | |
| Regulatory Status
|
|||



