Apolipoprotein B (Apo B)

 |
| Product Features
Diazyme's Apo B Assay has a linear range of 3.6 – 240 mg/dL with good correlation with existing FDA approved product with r2 values >0.99. The Diazyme Apo B Assay is not interfered by the following substances at the indicated concentrations: triglycerides at 1000 mg/dL, ascorbic acid at 10 μM, bilirubin at 40 mg/dL, bilirubin conjugate at 40 mg/dL, and hemoglobin at 1000 mg/dL. |
|||
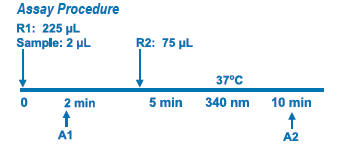 Assay Principle Assay Principle
This method is based on the reaction of a sample containing human Apo B and a specific antiserum to form an insoluble complex which can be measured turbidimetrically at 340 nm. By constructing a standard curve from the absorbance of standards the concentration of apo B in the samples can be determined. |
|||
| Intended use
The Diazyme Apolipoprotein B Assay is intended for the quantitative in vitro determination of Apolipoprotein B (Apo B) in serum. It can be used as an aid for assessing the risk of coronary artery disease. For in vitro diagnostic use. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ142A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ141A-Cal | Cal: 1 Level (Lyophilized) | |
| Control | DZ248A-Con | Con: 3 Level (Lyophilized) | |
| Regulatory Status
|
|||



