Sodium Assay

 |
| Product Features
Diazyme's Sodium Assay has good correlation with the ISE method and has a linear method between sodium concentrations of 80 and 180 mmole/L (184 and 414 mg/dL). The assay has CV's% of <2% with a correlation coefficient of 0.98, slope of 1.05 and a y intercept of -2.23. The following substances normally present in serum produced less than 10% deviation at the listed concentrations: NH4Cl at 1.5 mM, KPi at 2.0 mM, CaCl2 at 7.5 mM, KCl at 10 mM, CuCl2 at 0.5 mM, ZnCl2 at 0.5 mM, FeCl3 at 0.5 mM, Glucose at 5 mM, ascorbate 10 mM, bilirubin at 40 mg/dL, bilirubin conjugate 40 mg/dL, hemoglobin 500 mg/dL, and triglyceride 1000 mg/dL. |
|||
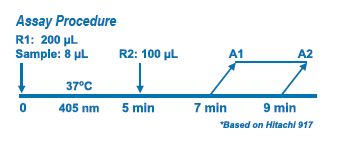 Assay Principle Assay Principle
Sodium is determined enzymatically via sodium-dependent β- galactosidase activity with ONPG as the substrate. The absorbance at 405 nm of the product O-nitrophenyl is proportional to the sodium concentration. |
|||
| Intended use
Diazyme Liquid Stable Enzymatic Sodium Assay is intended for the quantitative in vitro determination of sodium in serum. Measurements obtained by this device are used in the diagnosis and treatment of aldosteronism (excessive secretion of the hormone aldosterone), diabetes insipidus (chronic excretion of large amounts of dilute urine, accompanied by extreme thirst), adrenal hypertension, Addison's disease (caused by destruction of the adrenal glands), dehydration, inappropriate antidiuretic hormone secretion, or other diseases involving electrolyte imbalance. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ114B | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Enzymatic |
| Calibrator | DZ114B-Cal | Cal: 2 Level | |
| Control | DZ114B-Con | Con: 2 Level (Lyophilized) | |
| Regulatory Status
|
|||



