Procalcitonin (PCT) Assay

| Product Features Diazyme's Procalcitonin (PCT) Assay is for the quantitative determination of PCT in Serum, EDTA or Lithium Heparin plasma samples. PCT should be measured according to specific application parameters for specific chemistry analyzers. Diazyme's PCT Assay kit consists of two liquid stable reagents; R1, and R2, and the PCT calibrators and controls are packaged separately. Diazyme’s PCT methodology utilizes multiple monoclonal antibodies for enhanced assay sensitivity and specificity. |
||
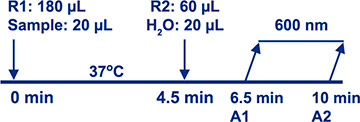 Assay Principle Assay Principle Diazyme's PCT Assay is based on a latex enhanced immunoturbidimetric method. PCT proteins in the sample bind to the specific anti-PCT antibody which is coated on latex particles, and causes agglutination. The degree of the turbidity caused by agglutination can be measured optically and is proportional to the amount of PCT in the sample. The chemistry analyzer calculates the PCT concentration of a sample by interpolation of the obtained signal of a calibration curve. The reaction is monitored at 600nm and completed within 10 minutes. |
||
| Intended use Diazyme PCT Assay is a latex particle enhanced immunoturbidimetric method intended for the quantitative determination of PCT in human serum, EDTA or lithium heparin plasma. Measurement of PCT in conjunction with other laboratory findings and clinical assessments aids in the risk assessment of critically ill patients on their first day of ICU admission for progression to severe sepsis and septic shock. For in vitro diagnostic use only. |
||
| Ordering Information | ||
| Product | Catalog Number | Format |
| Kit | DZ558A | R1/R2 (Dual Vial Liquid Stable, Immunoturbidimetric) |
| Calibrator | DZ558A-CAL | Cal: 6 Level |
| Control | DZ558A-CON | Con: 2 Level |
| Regulatory Status
|
||


