LDL-Cholesterol

 |
| Product Features
Diazyme's LDL-Cholesterol Assay is a direct dual liquid stable reagent system. Reagent transfer is eliminated for most chemistry systems with a choice of convenient instrument specific packaging options including Roche™ Hitachi 917 series, Beckman AU (400/600/640/680), Beckman Synchron CX, LX and DXC. The assay utilizes Diazyme's proprietary PVSPEGME coupled technology which provides outstanding performance. The assay features a linear range of up to 250 mg/dL, intra and inter %CV values < 5.0 % and less than 10% interference from triglycerides to 1000 mg/dL, ascorbic acid to 10 mmol/L, bilirubin to 40 mg/ dL, bilirubin conjugate to 30 mg/dL, hemoglobin to 1000 mg/dL. |
|||
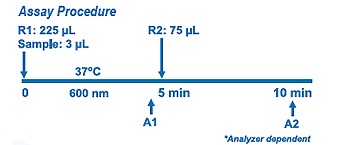 Assay Principle Assay Principle
The assay is based on a modified polyvinyl sulfonic acid (PVS) and polyethylene-glycol ether (PEGME) coupled classic precipitation method with the improvements in using optimized quantities of PVS/PEGME and selected detergents. LDL,VLDL, and chylomicron (CM) react with PVS and PEGME and the reaction results in inaccessibility of LDL, VLDL and CM by cholesterol oxidase (CHOD) and cholesterol esterase (CHER), whereas HDL reacts with the enzymes. Addition of R2 containing a specific detergent releases LDL from the PVS/PEGME complex. The released LDL reacts with the enzymes to produce H2O2 which is quantified by the Trinder reaction. |
|||
| Intended use
The Diazyme LDL-Cholesterol Assay is intended for the in vitro quantitative determination of Low Density Lipoprotein Cholesterol in human serum or plasma. The reagents can assist in the diagnosis and treatment of patients at risk of developing coronary heart disease. Elevated LDL cholesterol is the primary target of cholesterol-lowering therapy. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ128A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, PVSPEGME Coupled |
| Calibrator | DZ130A-Cal | Cal: 1 Level (Lyophilized) | |
| Control | DZ248A-Con | Con: 3 Level (Lyophilized) | |
| Regulatory Status
|
|||



