Glycated Serum Protein (Glycated Albumin)

| Product Features
Diazyme's enzymatic assay for glycated serum protein provides improved specificity and reliability compared to conventional NBT-based methods. The assay is highly precise with a total CV ≤ 1.3%. The assay offers extensive linearity up to 1354 μmol/L. The assay has no significant interference from: ascorbic acid (5 mg /dL); bilirubin (7.5 mg/dL); bilirubin conjugated (5 mg/dL); glucose (2400 mg/dL; hemoglobin (200 mg/dL); triglycerides (2000 mg/dL); and uric acid (35 mg/dL). |
|||
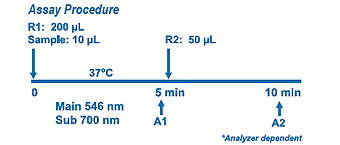 Assay Principle Assay Principle
Diazyme's Glycated Serum Protein Assay uses proteinase K to digest GSP into low molecular weight glycated protein fragments (GPF), and uses Diazyme's specific fructosaminase™, a microorganism originated amadoriase to catalyze the oxidative degradation of Amadori product GPF to yield PF or amino acids, glucosone and H2O2. The H2O2 released is measured by a colorimetric Trinder end-point reaction. The absorbance at 546-600 nm is proportional to the concentration of glycated serum proteins. |
|||
| Intended use
Diazyme Glycated Serum Protein Assay in conjunction with Diazyme Glycated Serum Protein single calibrator is intended for the quantitative determination of glycated serum proteins (GSP; glycated albumins; fructosamine) in serum. The measurement of glycated serum proteins is useful for monitoring diabetic patients. For in vitro diagnostic use only. |
|||
| Product | Catalog Number | Format | Method |
| Kit |
DZ112B | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Enzymatic |
| Calibrator | DZ112B-Cal | Cal: 2 Level (Lyophilized) | |
| Control | DZ112B-Con | Con: 2 Level (Lyophilized) | |
| Regulatory Status
|
|||




