Apolipoprotein A-I (Apo A-I)

 |
| Product Features
Diazyme's Apo A-I Assay kit has a linear range of 1.44 - 250 mg/dL and good correlation with an existing FDA approved product with r2 values >0.98. The Diazyme Apo A-I Assay is not interfered by the following substances at the indicated concentrations: triglycerides at 1000 mg/dL, ascorbic acid at 10 μM, bilirubin at 40 mg/dL, bilirubin conjugate at 40 mg/dL, and hemoglobin at 1000 mg/dL. |
|||
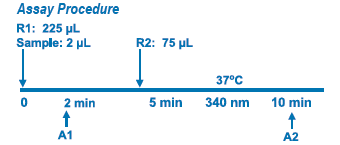 Assay Principle Assay Principle
This method is based on the reaction of a sample containing human apo A-I and a specific antiserum to form an insoluble complex which can be measured turbidimetrically at 340 nm. By constructing a standard curve from the absorbance of standards the concentration of apo A-I in the sample can be determined. |
|||
| Intended use
The Diazyme Apolipoprotein A-I Assay is intended for the in vitro quantitative determination of Apolipoprotein A-I (Apo A-I) in serum. It can be used as an aid for assessing the risk of coronary artery disease. For in vitro diagnostic use. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ141A | R1/R2 (Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ141A-Cal | Cal: 1 Level (Lyophilized) | |
| Control | DZ248A-Con | Con: 3 Level (Lyophilized) | |
| Regulatory Status
|
|||



