2-Reagent EZ Vitamin D Assay

Product Features
Diazyme's EZ Vitamin D Assay is the first and only 2-reagent, latex enhanced immunoturbidimetric assay for use on general clinical chemistry analyzers. This new Vitamin D assay allows many clinical laboratories to run the EZ Vitamin D test in house, at a cost effective price, without the need for special instrumentation. The EZ Vitamin D assay is fully automated requiring no sample pre-treatment or pre-dilution steps and recognizes both 25-OH Vitamin D2 and D3 equally. Diazyme’s EZ Vitamin D assay ensures high assay throughput, precision CV of <10% and a linear range from 7.6 - 147.8 ng/mL. Diazyme’s EZ Vitamin D assay is FDA-cleared and CE marked, with results correlating to the LC-MS/ MS method. |
|||
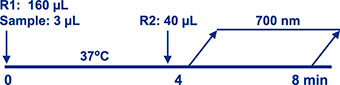 Assay Principle Assay Principle
The Diazyme EZ Vitamin D Assay is a direct particle-enhanced immunoturbidimetric assay. The assay’s proprietary reagents are designed to dissociate vitamin D from vitamin D binding proteins, found in serum or plasma specimens, while particles coated with anti-vitamin D antibodies bind to the dissociated vitamin D, thereby causing agglutination. This agglutination is detected as an absorbance change (700 nm), with the magnitude of the change being proportional to the quantity of total vitamin D in the sample. Specimen concentrations are determined by interpolation from a 5-point calibration curve prepared from calibrators of known concentrations. |
|||
| Intended use
The Diazyme EZ Vitamin D assay is intended for use in clinical laboratories for the quantitative determination of 25-OH vitamin D (vitamin D) in human serum and plasma, using automated chemistry analyzers. Measurement of vitamin D is used for the assessment of the vitamin D sufficiency. For in vitro diagnostic use only. |
|||
| Product | Catalog Number | Format | Method |
| Kit | DZ888A | R1/R2(Dual Vial Liquid Stable) | Dual Vial Liquid Stable, Immunoturbidimetric |
| Calibrator | DZ888A-Cal | Cal: 5 Level | |
| Control | DZ888A-Con | Con: 2 Level | |
| Regulatory Status
|
|||




